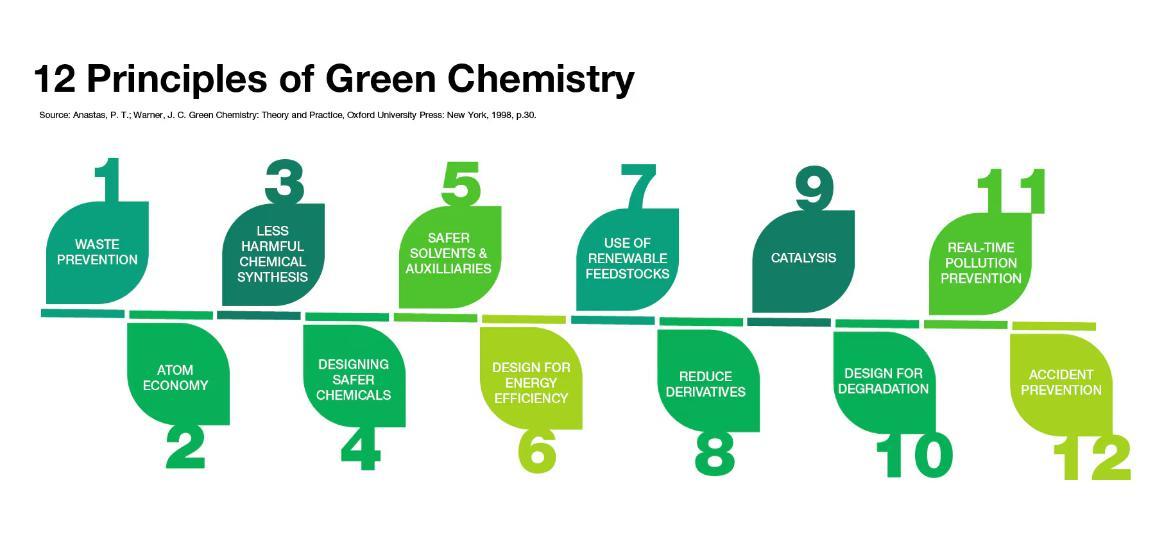
1. Waste Prevention
This principle is about preventing waste wherever and whenever possible before creating it. This includes waste generated throughout the process as well as chemical waste that will need special treatment, cleaning, or disposal at the end.
2. Atom Economy
In lay terms, atom economy is integrating as much of the materials used as possible into the end product - using most, if not all, of the atoms! In other words, atom economy is a measure of reaction efficiency. This will look different depending on which field you're in, but the principle is the same. Tailor your experiments and processes to use less materials and to have less or no by-products.

3. Less Hazardous Chemical Synthesis
Where possible, design reactions that are as safe as possible. This includes hazards from reactants, products, by-products, and waste generated. Consider how the hazards may affect you, others around you, and the environment.
4. Designing Safer Chemicals
Design chemicals that are less toxic (and require less toxic raw materials) while maintaining their function and efficacy.
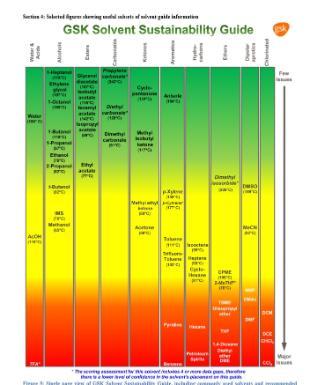
5. Safer Solvents and Auxiliaries
Minimise use of solvents and auxiliary substances (drying agents, packing agents, surfactants, preservatives, stabilisers etc.). Otherwise, use safer options where total removal isn't possible.
A green or greener solvent is a great way to achieve this. Check out our “Small Steps to Green Chemistry” and “Resources” for more information.
6. Design for Energy Efficiency
When designing experiments and processes it is important to recognize and minimize how much energy will be used in the process. One way to do so is to carry out reactions at ambient temperature and pressure.
The way this can be achieved will highly depend on the process, but some ways include using microorganisms for organic synthesis, using renewable materials / energy, or improving the technology of heating systems.
7. Use of Renewable Feedstocks
When purchasing chemicals and materials it is important to ensure that they are made from renewable sources / feedstocks. Using materials that are renewable, will ensure that there isn't pressure put on the environment but also ensure that future generations have access to these materials.
Some examples of this principle include the use of microbial or plant biomass such as the production of biodiesel, bioethanol and butanol from sugars and lignocellulose.
8. Reduce Derivatives
Derivatives, like protecting or blocking groups, are frequently used in synthesis to facilitate a reaction. However, these extra groups require extra steps and lead to waste.
One example of this is using enzymes during the synthesis process which eliminates the need for protecting groups due to their specificity.
9. Catalysis
Reagents are used frequently in facilitating chemical reactions. Two widely used types include stoichiometric (used up in a reaction) and catalytic (participate without being used up / consumed) reagents.
While stoichiometric reagents are simpler and more convenient to use, they are usually consumed in large amounts leading to a higher long term cost and excess waste. Catalytic reagents are a greener alternative as they are used in smaller amounts and can be reused or regenerated leading to a lot less waste.
For a more in depth description of this principle check out the University of Toronto’s Green Chemistry Initiative describe it in their(opens in a new window)Green Chemistry video series.
10. Design for Degradation
For those who design chemicals or chemical processes - design for any products or byproducts to break down into non-hazardous / non-toxic substances and ensure that they do not accumulate in the environment.
11. Real-Time Pollution Prevention
When designing and carrying out processes ensure that there is a way to monitor and control any formation of byproducts or other materials that can lead to pollution of the environment. This includes the previous principle as well as monitoring the use of plastics, fossil fuels, and overall carbon footprint.
A simple way to start this is to ensure that you are using a local supplier / manufacturer. Depending on the supplier, you may also be able to find or request a Certificate of Origin that states where the product originated to further reduce your footprint. Similarly, studying the product's Safety Data Sheets is important to ensure that there is little to no release of the product into the environment during use. Other options include swapping to glass vials and pipettes or even rewashing plasticpipetteswhere possible.
12. Accident Prevention
Design chemicals and processes that include accident prevention. This includes ensuring that the substances used are safe to use and as non-toxic as possible. Similarly, study the Safety Data Sheets to ensure correct storage and handling as well as the product's stability over time.


 Green Chemistry
Green Chemistry