Tuesday, 21 January, 2025
Researcher: Dr Jufan Zhang, UCD School of Mechanical and Materials Engineering
Summary
Needle phobia is an intense fear of needles in medical settings and can lead people to avoid or delay medical care. It affects over 10% of the global population, including the majority of children (50%-60%). In Ireland, 11% of 9-year-old children have a chronic illness or disability, for whom the safe administration of injectable medicines at home would make a significant difference to their care. Dr Jufan Zhang’s team develops user-friendly microneedle devices for easy self-administration of medications carried out by the family of the patient. This minimises pain for children, and significantly reduces their fear of receiving medication by injection. This research has led to two grants from Enterprise Ireland, the establishment of a spin-out company, four industrial collaborations, a filed patent, four types of products, and several invited conference talks and personal awards for Dr Zhang.
Research description
Current injection devices normally use long needles (e.g. 25-38 mm for intramuscular administration), resulting in significant risks such as needle-stick injury, pain, needle phobia, or unsafe disposal. This practice prevents millions of patients worldwide from administering various applicable drugs themselves. Dr Zhang uses miniaturised needles to solve these problems without compromising the efficacy of the drug for the patient.
Since 2020, Dr Zhang has developed smart microneedle devices to enable the self-administration of medicines anywhere, anytime, by anybody, especially facilitating the medical care of children at home by their families. Supported by two Enterprise Ireland grants and four industrial partners since 2022, Dr Zhang has organised a team comprising multiple dedicated postdoctoral researchers and research assistants to develop four devices to address different targeted therapeutic requirements.
These devices include:
- Hollow microneedle inserts for a quick and simple connection to standard medical syringes, replacing long needles to mitigate pain and fear in sick children;
- Wearable devices for digitalised injection of larger dosage, allowing paediatric care at home in a safe and easy way;
- Dissolving microneedle patches for controllable slow release of medication, especially suitable for healing wounds, local pain release and skin disease that is common in children;
- An automatic microneedle-roller for facial cosmetic applications.
Dr Zhang’s research overcomes two key barriers in the microneedle market - limited dosage capacity and unsatisfactory mass manufacturing. His devices can deliver up to 2ml of liquid drug in a limited time, with the potential to deliver even more. His manufacturing solution costs less than €2 per microneedle insert to produce. This is low enough to support disposable use and does not create additional burdens to users for sterilisation. The microneedles are also recyclable.
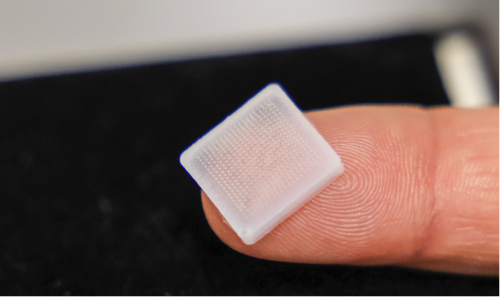
Research impact
Health and social impact
Over 90% of pre-school children and 50% of primary school children experience severe distress during vaccinations due to a fear of needles, causing many to delay receiving vaccinations. Needle phobia significantly impedes children in getting necessary medical care, not only for vaccinations, but also for blood tests and injections, amongst other treatments. There are about 131,764 children with a disability in Ireland (which accounts for 10.8% of the entire population of children) who could significantly benefit from receiving healthcare at home with microneedles.
As well as benefitting children, (opens in a new window)it was reported that avoidance of the influenza vaccination because of fear of needles occurred in 16% of adult patients, 27% of hospital employees, 18% of workers at long-term care facilities, and 8% of healthcare workers in hospitals. Dr. Zhang’s partners have tested his microneedle devices and proven that users experience little pain and have minimal invasion on the skin and the microneedles significantly mitigate the stresses and concerns of users as well as leading to better efficacy for some medical treatment.
This method of administering medication has the potential to significantly benefit the 1,109,557 people (22% of Irish population) reported in the (opens in a new window)2022 Census of having a long-term condition or disability, who can now be treated safely and comfortably in their own homes by their own families or support network.
This has directly facilitated the collaboration with Huana Biotech in exploring blood sampling applications. Thanks to his industrial partners’ international sales network, Dr. Zhang will be deploying the microneedle devices to multiple regions around the world, including the US, China, the UK and Spain. A spin-out company will specifically handle these businesses and the deployment of microneedles, beginning in 2025.
Dr. Zhang’s technology has overcome key barriers in the market and brought genuine value to the healthcare industry. We are interested in distributing his products through our sales network and looking forward to a viable collaboration model, such as a joint venture or IP licensing.
— CEO of Huana Biotech
Economic impact
There is currently only a limited number of microneedle devices delivered to the market commercially. Dr. Zhang’s technology tackles two main issues in the market– the small applicable dosage from the microneedles that are currently available and the high production cost of these microneedles. This has attracted multiple multinational and Irish companies to collaborate on tailored solutions for delivering plasma proteins, gene therapy and cosmetics with microneedles. Branca Bunus has estimated a demand for over 100,000 components of these devices annually, and foresees a minimum annual turnover of €1.5-€2 million. Enterprise Ireland has awarded two grants to this research for establishing a spin-out company in Ireland.
Academic impact
Dr Zhang was ranked (opens in a new window)Top Influential Leaders Making Waves in the Manufacturing Industry to Know in 2024 by InsightsSuccess Magazine. His Enterprise Ireland Commercialisation Fund was among the top-funded projects of its category in UCD. He is one of a limited number of researchers capable of accurately monitoring and tracing the microneedle-skin-liquid interactions. This has attracted multiple academic and industrial collaborations already. Additionally, Dr Zhang has been invited 24 times in the last two years to give talks at international conferences and workshops due to his featured research on innovative medical devices.
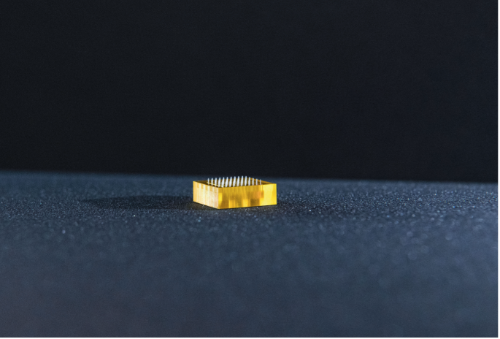
Educational impact
This project has trained three postdoctoral researchers and offered industrial collaboration opportunities with different companies for each one. This strongly supports the career development of these early-stage researchers. Furthermore, Dr Zhang is the regional lead (Ireland & the UK) of a European engineering educational program – (opens in a new window)YouLead, which has provided training to 40 young entrepreneurs.
Technological impact
Dr. Zhang’s microneedle technology has been praised by over 10 companies worldwide for advancing the state-of-the-art technology and filling essential gaps (particularly regarding dosage, cost and ease of use) in the market.
Kestrel Technology Consulting UK has completed a market investigation report which proved that 15 therapeutic areas directly benefit from Dr. Zhang’s devices, all confirmed by industrial contacts. Some prominent therapies such the administration of growth hormones, vaccines and immunotherapy are especially essential for children and will be made much easier with the use of microneedles.
Among the most impressive aspects of the technology is the low production cost of just €2 per microneedle insert. Dr. Zhang has filed an international patent and is disclosing another one for a wearable device to administer medication.
A senior director at Abbvie said that “UCD’s microneedles solution, based on the points about low cost, flexibility, the benefits of self-administration etc. could provide a real clinical benefit in some specific areas”.

