How protein protection mechanisms aid breast cancer cell migration and other stories from our latest publications
Breast cancer is the leading cause of cancer death in women worldwide. In Ireland, breast cancer has become the second most common cancer with over 2,000 women diagnosed every year. With an estimated one in eight women affected by breast cancer in their lifetime, scientists are urgently trying to find new diagnostic and therapeutic strategies to combat this disease and improve patient outcome.
Breast cancers are often clinically classified based on the type of receptors they bear. These are proteins that reside on the cell surface responsible for relaying extracellular signals into the cell to trigger a responsive chain of events. A substantial proportion of breast cancer patients display high level of members of a receptor family called ErbB, which consists of four related receptors EGFR, Her2, Her3 and ErbB4. The ErbB receptor network has thus received a lot of attention from researchers and has been a major therapeutic target in breast cancer. Despite significant progress in the development of drugs, particularly those targeting EGFR and Her2, patients always experience relapse of the disease after an encouraging, but short, initial response.
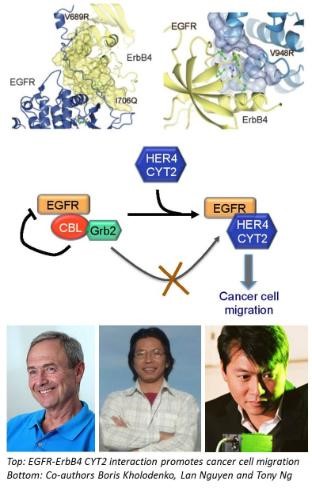
In a collaborative study recently published in the leading international journal Science Signalling, SBI researchers and co-authors from King's College London, University of Zurich, The Weizmann Institute of Science (and others) have worked out another important piece of the ErbB puzzle and shed light on how the ErbBs receptors interact with each other to influence how breast cancer cells move. They found that a form of the receptor ErbB4, called CYT2, could protect its kin protein EGFR from being destroyed by the cellular destruction machinery by shielding EGFR from a destroyer protein known as E3 ligases. This protection mechanism helps breast cancer cells migrate more efficiently.
“This piece of mechanistic understanding allowed us to better map out the ErbB interactions to construct a predictive mathematical model for the network. The model enables us then to develop a systems-level view of how the network behaves under various perturbations even before we go to the lab”, said SBI Research Fellow Dr Lan Nguyen and Prof. Boris Kholodenko who were involved in the research.
“Our findings uncovered novel mechanisms that may influence the efficacy of EGFR-targeted therapy. Moreover, the effect of ErbB4 CYT2 on cell motility – a key feature enabling cancer cells invasion and metastasis – suggests that it could be a potential therapeutic target” said Prof. Tony Ng from King's College London who led the study.
Further information:
The ErbB4 CYT2 variant protects EGFR from ligand-induced degradation to enhance cancer cell motility.
Kiuchi T, Ortiz-Zapater E, Monypenny J, Matthews DR, Nguyen LK, Barbeau J, Coban O, Lawler K, Burford B, Rolfe DJ, de Rinaldis E, Dafou D, Simpson MA, Woodman N, Pinder S, Gillett CE, Devauges V, Poland SP, Fruhwirth G, Marra P, Boersma YL, Plückthun A, Gullick WJ, Yarden Y, Santis G, Winn M, Kholodenko BN, Martin-Fernandez ML, Parker P, Tutt A, Ameer-Beg SM, Ng T. Sci Signal. 2014 Aug 19;7(339):ra78. doi: 10.1126/scisignal.2005157.

