From bench to bedside: the research behind falling cancer mortality rates
Earlier this week, Cancer Research UK released their annual mortality statistics which reveal that deaths from the four most prevalent cancers in the UK have fallen by a third over the last 20 years, while the National Cancer Registry of Ireland’s figures reveal a similar a trend. We ask two leading breast cancer specialists from UCD to explain the science behind the advances that have been made, what we can expect cancer research to deliver in the next 10 years and what role a systems biology approach has to play.
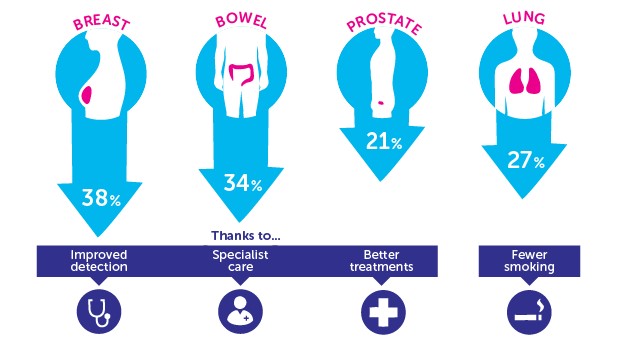
Infographic showing fall in mortality rates over the past 20 years for the UK's four most prevalent cancers (courtesy of Cancer Research UK)
Key to the reductions in cancer-related deaths that the Cancer Research UK statistics have highlighted are the enormous strides made in ‘targeted therapies'. This approach means that cancer cell growth is blocked by targeting specific molecules in the cell that are needed to grow and survive. We are also seeing the emergence of affordable and widespread molecular diagnostics, where a tumour can be profiled and the cancer treatment tailored to that tumour.
Although significant advances have been made in scientific research, we are still lacking in effective anti-cancer drugs. Translating discoveries from the lab bench to the clinic can be a very slow process, and chemotherapy and targeted therapies aren’t without their problems. Tumours can evolve and become resistant to treatment, causing them to reoccur and spread to other parts of the body.
Prof. William Gallager is Professor of Cancer Biology in UCD, where he has established the Cancer Biology and Therapeutics Laboratory, is co-founder of OncoMark and is Director of the Irish Cancer Society Collaborative Cancer Research Centre BREAST-PREDICT. SBI Research Fellow Dr Alex von Kriegsheim, formerly of the Beatson Institute for Cancer Research in Glasgow, uses systems approaches to understand how modifications to proteins drive the progression and spread of cancer. Alex has recently been awarded a £200,000 research grant by Breast Cancer Campaign, with Prof. Gallagher as co-applicant, to study the role played by a small protein in breast cancer metastasis.
What advances in breast cancer research over the past 20 years have been behind this fall in mortality rates?
William Gallagher: There has been a sea-change in how breast cancer is managed clinically over the last couple of decades, spear-headed by the development of more targeted therapies. The' poster-child' of such treatments is Herceptin, which is a monoclonal antibody that targets the HER2 protein which is over-expressed in approximately one third of breast tumours. Patients with such HER2 positive breast cancers, prior to the development of Herceptin, had the expectation of a generally very poor outcome; this has dramatically changed since the arrival of Herceptin and similar HER2-targeted therapies, such that long-term survival is commonly seen, albeit with emergency of resistance still a pressing issue.
Alex von Kriegsheim: Aside from novel treatments we should not forget that advances in early detection have played a major role in reducing cancer mortality. When diagnosed early, the survival rates are generally higher and treatment options are broader. Efficient and predictive molecular diagnostics, which will allow the detection of cancers even when asymptomatic, are currently in development. If successful such assays would catch the cancers at an earlier stage, thus reducing mortality even further.
 |
"Advances in early detection have played a major role in reducing cancer mortality. When diagnosed early, the survival rates are generally higher and treatment options are broader." Alex von Kriegsheim |
|---|
What does this mean for a targeted approach in other cancers?
WG: Spurred on by other successful targeted therapies, the drug development pipelines in large pharma and biotech companies have brought forward multiple novel targeted agents that tackle specific defects or abnormalities in breast and other cancers. Several of these novel treatments have been approved for clinical use in breast cancer, with multiple others on the cusp of being so. Together with advances in diagnostics, this is where the real progress has been made in changing the clinical perspective of breast cancer patients.
Where do you see breast cancer research going in the next 5-10 years?
WG: While we have made great in-roads into understanding some of the basic molecular underpinnings of breast cancer, the conversion of the wealth of information that is available into clinical reality has not kept up to pace, notwithstanding the above-mentioned exceptions. Critically, a key issue with both conventional therapy and more targeted agents is the evolution of tumours over time to become resistant to treatment. One of the key focuses of the Irish Cancer Society BREAST-PREDICT Collaborative Cancer Research Centre, as well as some EU-funded programmes I am involved with, relates to uncovering the mechanisms underlying such resistance phenomena via the use of an integrated molecular profiling approach, together with advanced bioinformatic analysis.
Importantly, our research is greatly advanced by access to longitudinally acquired samples obtained generously from breast cancer patients throughout their treatment course. Such individualised series of biological specimens are invaluable in identifying what happens to tumours during treatment. Another crucial aspect of our approach is the close interplay between computational and experimental biologists and those in the clinic that are directly managing breast cancer patients, such as surgeons and oncologists. This interdisciplinary, ‘systems’ approach is key to translating our basic discoveries to clinical impact.
|
"A key issue with both conventional therapy and more targeted agents is the evolution of tumours over time to become resistant to treatment." William Gallagher |
 |
|---|
AVK: William has highlighted one of the aspects which will further reduce cancer mortality in the future. Breast cancer, is like most cancers, a heterogeneous disease and drugs like Herceptin are targeted at the significant subsection of patients which express HER2. Right now, in depth sequencing of the patient and cancer-specific mutations are on the verge of becoming affordable and widespread. These data can then be analysed and fed into models which predict the treatment outcome and will allow designing discreet and patient-orientated regimes, increasing the efficiency and affordability of the treatment.
In addition, we still require treatments to prevent the cancer from spreading to secondary sites, which ultimately leads to the patient’s death. We are currently starting to understand why and how cancer spreads. My own research, funded by Breast Cancer Campaign and developed in conjunction with William and colleagues from SBI and UCD Conway Institute, utilises systems biology techniques to focus on the role played by a small protein in the spread of breast cancer to other parts of the body. We hope that over the next 5-10 years research into these biological processes will yield new strategies to prevent this spread, or metastasis, from occurring, bringing further significant reductions in cancer mortality rates.
Carolanne Doherty is a PhD researcher at SBI whose work focuses on signalling of the MST2/LATS1 pathway which is altered in many human cancers, in particular a protein called YAP which can act as a tumour suppressor or an oncogene.

