Breaking Down Childhood Neuroblastoma: Searching for New Ways to Treat the Disease
I am a postdoctoral fellow at System Biology Ireland (SBI), and I am currently working on a research project that is funded by DevelopMed, a Marie Sklodowska-Curie (MSCA) COFUND Action. DevelopMed is an international training and Career Development Fellowship Programme for the duration of three years, led by University College Dublin.
My research focuses on a type of cancer called Neuroblastoma (NB), which has been a main topic of study at System Biology Ireland for many years. However, my current research project in this field has been ongoing for less than a year. In this blog, I aim to present my scientific research for a broad audience to help readers understand our research focus and why we are looking to open a new angle in the study of NB.
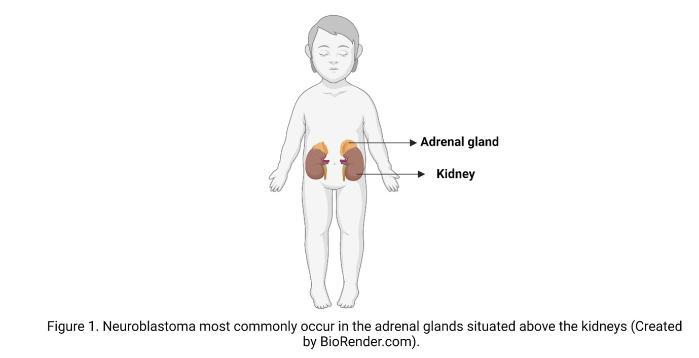
A cancer diagnosis is always a shock, but in the case of children, it is especially devastating. After brain cancer, NB is the most common solid tumour in children, and despite its name does not originate in neurons of the brain but from the immature nerve cells in the adrenal glands or other peripheral parts of the nervous system. The way the disease progresses is different from patient to patient, and it can range from good to poor depending on various factors. These factors include the age of the child, the location of the tumour and how advanced it is when detected, and the changes in the DNA, or what we call the “genetic makeup” of the cancer cells.
In low and intermediate cases, patients have very good prognosis and respond well to the treatment available in the clinic, with more than 90-95 percent having a 5-year survival rate. Unfortunately, the group of children who have a high-risk tumour currently have a low survival with less of 40 percent survival rate after 5 years. Even if they are cured, high risk patients need very aggressive treatments that can have severe short and long-term side effects. For these reasons a lot of efforts are currently dedicated to identifying treatments that have fewer side effects but are more effective for all the patients. This requires us to get a better understanding of the disease.
In my project we want to extend our understanding of the mechanisms that contribute to the development of the disease and relapse. In scientific terms, my research addresses this clinical need by studying the cross-communication between signalling pathways in high-risk/relapsed NB.
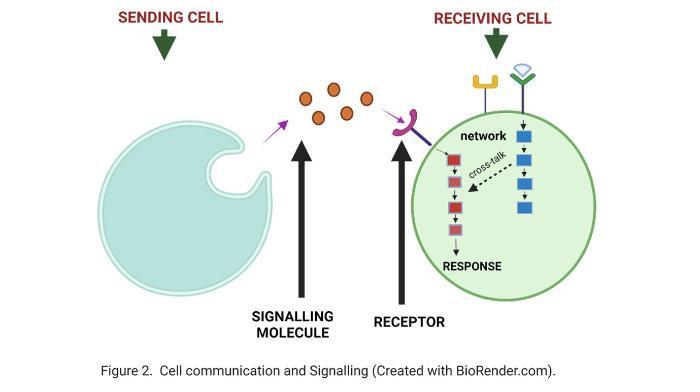
So, what is cell signalling? Cell signalling is a critical process that enables cells to communicate with each other and within themselves. Just as how a road network connects different locations, the signalling network in a cell is formed by “roads” connected with each other and we call an individual “road” a signalling pathway. These pathways are fundamental to proper functioning of the cell and body. In diseases such as cancer, this network is broken, leading to cells dividing when they should not, or surviving when they should die. Fixing this broken network is the key to developing effective treatments and this is what I try to do with my research. Today we have a good idea of the main pathways that are regulating the normal work of a cell, and which are the ones that are often broken in cancers. Researchers at SBI are studying ways to fix the ERK pathway for over 30 years, and it is a significant pathway in our cell’s signalling network as a potential treatment for cancer. The pathway is deregulated in approximately 40 percent of all human cancer. The reason why this is important in NB is that we have data showing that the hyperactivation of the ERK signalling pathway occurs in 80 percent of cases of high-risk and relapsed NB, indicating its significance in these cases. However, we also know from many cancers that targeting this pathway alone does not fix the broken communication. This is because, as it happens in a road network, targeting one block of a highway is like blocking one signalling pathway; the cell can find secondary roads to get from point A to point B. In cancers, stopping the signal from the ERK pathway can push the tumour cell to use other pathways that ultimately allow the cell to survive the treatment.
One way to get more effective cancer treatments is to stop the connections of this pathway with other pathways that act as a bypass, which we call “crosstalk points”. One of the alternative pathways is called the MST2 signalling pathway and the crosstalk between the ERK and MST2 pathways is the focus of my research.
The MST2 pathway has the opposite function in the cell than the ERK pathway as it tells the cell when it has to die and therefore prevents tumour growth depending on the tissue and signalling context. My supervisors in SBI have been studying the MST2 pathway in other cancer types for over more than 20 years. We know that in cancer one of the bad effects of the activation of the ERK pathway is that it stops the MST2 pathway, and this prevents the cell from dying. The MST2 pathway is very complex, and we are still mapping it. What we know is that it consists of various proteins that do not work properly in cancer cells due to changes in the expression or activity of its core components, and it has been implicated in various cancers including NB. Among the proteins of this pathway, we have MST2 and LATS1 that are at the centre. Another protein called RASSF1A usually helps to stop tumours from growing, but in cancer, the expression of this gene is often lost because of something called promoter hypermethylation.
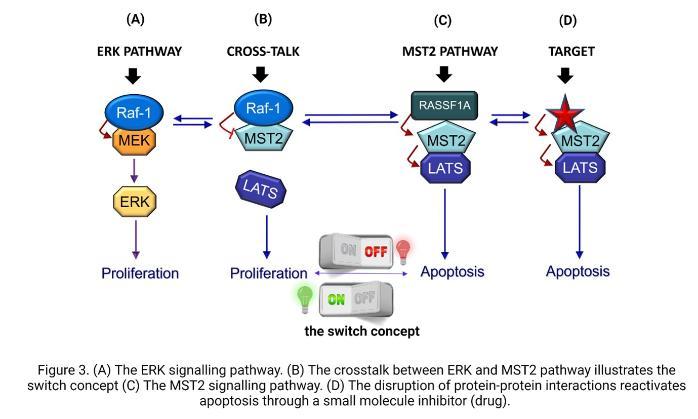
This is also true in neuroblastoma, where RASSF1A is methylated in 70 percent of cases. Scientists found that this gene is even more likely to be lost in tumours that have a specific genetic feature called MYCN amplification in NB. These MYCN proteins are usually expressed at a very high level in those patients who are high risk and often show poor therapy responses and disease relapse. This information brings together MYCN, one of the proteins that we know is very important in NB, and RASSF1A that is a key regulator of the MST2 pathway. However, no one has yet looked at the activation status of the MST2 pathway core components in these tumours and how they are affected in high-risk NB. We believe that the connection between the ERK pathway and the MST2 pathway is disrupted in NB cells, and that restoring this connection could be beneficial. But how do these pathways communicate with each other? There are different ways they can interact, and they can affect the behaviour of the cell, either promoting growth and division (proliferation) or triggering cell death (apoptosis). This is akin to a switch that is composed of two proteins, RAF1 and MST2 (as shown in Figure 3).
These proteins connect both pathways and the switch can turn on cell growth and multiplication (proliferation) or turn off the process and trigger cell death (apoptosis). However, sometimes the switch can get stuck in the "on" position, causing uncontrolled cell growth and leading to tumour formation. Therefore, my research aims to investigate the RAF1-MST2 crosstalk, a communication process that inhibits the cell death signal mediated by the MST2 pathway and explore if targeting this cross-communication can make the switch work again and bring back the cell death signal. If we see this is the case, we think that in the future this could provide a new therapeutic strategy for high-risk or relapsed NB patients. But it is important to stress that we are just at the first steps of the project.
To achieve this goal, I first compare the RAF1-MST2 crosstalk in NB cells that we can grow in the lab. There are two types of cells that I use: one that has a low level of MYCN and others that have high levels. This allows as to see what the status of the switch in these cells is and if it is working or not. We have some encouraging results from our initial experiments, but we need to further confirm our findings. Next, what we are doing is “drawing” the map of the signalling network involving ERK and MST2 in these cells, using mass spectrometry-based proteomics. Mass Spectrometry is a complex and powerful technique that allows us to identify and quantify proteins and their connections in a sample. Basically, it is like fishing with bait, a technique that is well explained in a previous SBI blog. Having this map will help us to understand better why the cells are not behaving properly. With this information, we can select a panel of key proteins that regulate the switch between cell growth and cell death and see if any of them are potential targets for treating NB patients.
Reactivating the normal balance between the ERK and MST2 pathways is not going to be an easy task, but we hope that ultimately it could offer new therapeutic options for NB patients who currently have no treatment. I will keep you posted about the progress of this project!!
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 945425, and from Science Foundation Ireland (SFI) and the National Children's Research Centre / Children’s Health Ireland under Grant Number 18/SPP/3522 (POI). The opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of POI and European Union’s Horizon 2020 research and innovation programme.
Scientific publication references for further reading:
- Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity.
Lee S, Rauch J, Kolch W. Int J Mol Sci 21(3):1102, 2020. - Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Romano, D…Kolch, W. Nature Cell Biology 16: 673, 2014.
- Competing to coordinate cell fate decisions: the MST2-Raf-1 signaling device. Nguyen K Matallanas D....Kolch W. Cell Cycle 14:2,189, 2015.

About the author
Rashmi Sharma is postdoctoral researcher in the Kolch and Gomez-Matallanas Groups at SBI. She is also a DevelopMed Fellow.

